Iodine And Zinc Balanced Equation . Web zinc + iodine = zinc iodide. Zinc powder is added to a solution of iodine in ethanol. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Web zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to form one mole of zinc iodide [zni. Web using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Web zinc iodide = zinc + iodine. Zn (s) + i 2 (s) → no reaction In this experiment, you will react roughly equivalent masses of zinc and iodine to yield a simple binary compound. This can be reversed using electrolysis to decompose the compound. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis.
from www.numerade.com
Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Zinc powder is added to a solution of iodine in ethanol. Web zinc iodide = zinc + iodine. Zn (s) + i 2 (s) → no reaction Web zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to form one mole of zinc iodide [zni. This can be reversed using electrolysis to decompose the compound. In this experiment, you will react roughly equivalent masses of zinc and iodine to yield a simple binary compound. Web using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis.
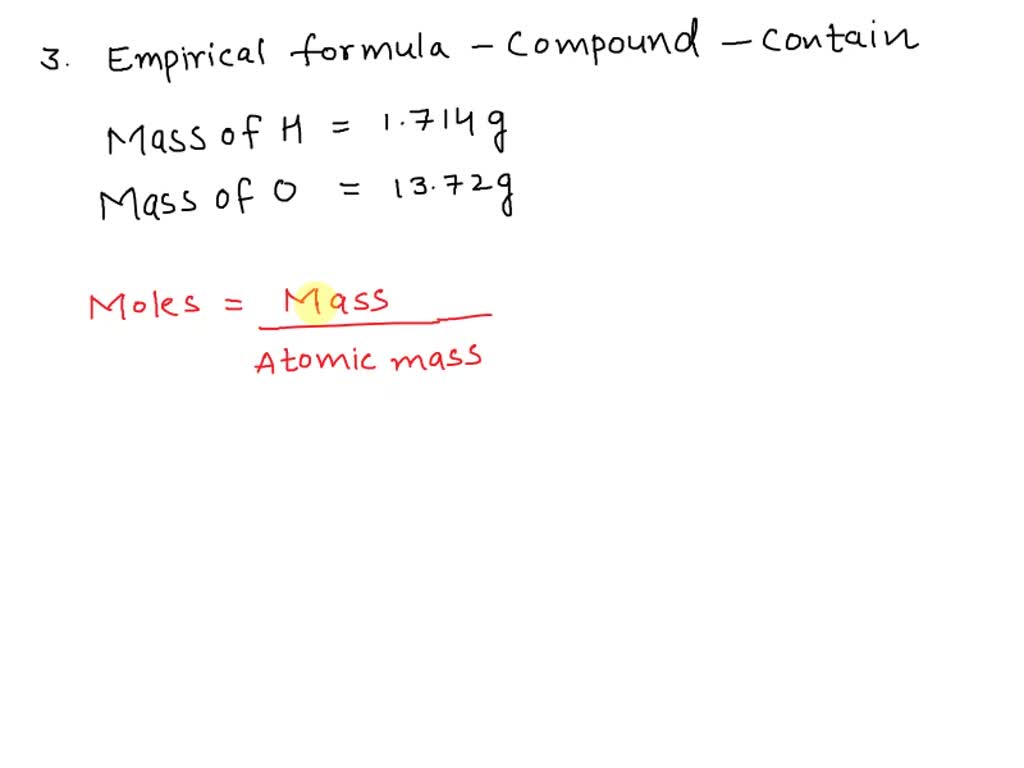
SOLVED What is the expected empirical formula for the compound that
Iodine And Zinc Balanced Equation Web zinc iodide = zinc + iodine. Zn (s) + i 2 (s) → no reaction Web using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Web zinc iodide = zinc + iodine. Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Web zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to form one mole of zinc iodide [zni. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. This can be reversed using electrolysis to decompose the compound. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. Web zinc + iodine = zinc iodide. Zinc powder is added to a solution of iodine in ethanol. In this experiment, you will react roughly equivalent masses of zinc and iodine to yield a simple binary compound.
From www.chegg.com
Solved Finding the Empirical Formula of Zinc Iodide Report Iodine And Zinc Balanced Equation In this experiment, you will react roughly equivalent masses of zinc and iodine to yield a simple binary compound. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. Zinc powder is added to a solution of iodine in ethanol. Web zinc + iodine = zinc iodide. Zn (s) + i 2. Iodine And Zinc Balanced Equation.
From www.numerade.com
SOLVED The balanced chemical equation for the reaction between Iodine And Zinc Balanced Equation Web zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to form one mole of zinc iodide [zni. Zn (s) + i 2 (s) → no reaction Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved. Iodine And Zinc Balanced Equation.
From www.numerade.com
SOLVED Text Writing and Balancing Chemical Equations Write balanced Iodine And Zinc Balanced Equation Zinc powder is added to a solution of iodine in ethanol. Web using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers. Iodine And Zinc Balanced Equation.
From www.numerade.com
SOLVED A Synthesis and formula of zinc iodide 1 Write the expected Iodine And Zinc Balanced Equation Zinc powder is added to a solution of iodine in ethanol. Web zinc + iodine = zinc iodide. Web zinc iodide = zinc + iodine. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. This can be reversed using electrolysis to decompose the compound. Web the chemical equation described in section. Iodine And Zinc Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced net ionic equation for each of the following Iodine And Zinc Balanced Equation Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. This can be reversed using electrolysis to decompose the compound. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. Web zn + i = zni2 is a synthesis reaction. Iodine And Zinc Balanced Equation.
From www.meritnation.com
write a balanced chemical equation for the reaction between zinc and Iodine And Zinc Balanced Equation This can be reversed using electrolysis to decompose the compound. Zinc powder is added to a solution of iodine in ethanol. Zn (s) + i 2 (s) → no reaction Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Web zinc iodide = zinc + iodine. Web. Iodine And Zinc Balanced Equation.
From exotwicqs.blob.core.windows.net
Iodine And Zinc Equation at Nancy Moore blog Iodine And Zinc Balanced Equation Web using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. In this experiment, you will react roughly equivalent masses of zinc and iodine to yield a simple binary compound. This can be reversed using electrolysis to decompose the compound. Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms. Iodine And Zinc Balanced Equation.
From www.numerade.com
SOLVED Aqueous copper(II) ion reacts with aqueous iodide ion to yield Iodine And Zinc Balanced Equation Web zinc + iodine = zinc iodide. In this experiment, you will react roughly equivalent masses of zinc and iodine to yield a simple binary compound. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. Zinc powder is added to a solution of iodine in ethanol. This can be reversed using. Iodine And Zinc Balanced Equation.
From www.numerade.com
SOLVEDSynthesis and formula of zine iodide WVrite the expected Iodine And Zinc Balanced Equation Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. Web zinc + iodine = zinc iodide. Zni2 = zn + i is a decomposition reaction where one mole of. Iodine And Zinc Balanced Equation.
From www.numerade.com
SOLVED Zinc reacts with iodine in a synthesis reaction. Using a Iodine And Zinc Balanced Equation Web zinc + iodine = zinc iodide. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. This can be reversed using electrolysis to decompose the compound. Zn (s) + i 2 (s) → no reaction Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide. Iodine And Zinc Balanced Equation.
From www.toppr.com
Identify the type of reactions taking place in each of the following Iodine And Zinc Balanced Equation Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Zinc powder is added to a solution of iodine in ethanol. In this experiment, you will react roughly equivalent masses of zinc and iodine to yield a simple binary compound. Web the reaction of zinc metal with iodine. Iodine And Zinc Balanced Equation.
From edu.rsc.org
Exothermic redox reaction of zinc with iodine Experiment RSC Education Iodine And Zinc Balanced Equation Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Web using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. Web the chemical equation described in section. Iodine And Zinc Balanced Equation.
From www.toppr.com
Write balanced chemical equations the following word equationZinc Iodine And Zinc Balanced Equation In this experiment, you will react roughly equivalent masses of zinc and iodine to yield a simple binary compound. Zn (s) + i 2 (s) → no reaction Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Web zinc iodide = zinc + iodine. Zinc powder is. Iodine And Zinc Balanced Equation.
From www.toppr.com
In the reaction between zinc and iodine , zinc iodide is formed . What Iodine And Zinc Balanced Equation Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. Zinc powder is added to a solution of iodine in ethanol. Web zinc iodide = zinc + iodine. Web zinc + iodine = zinc iodide. Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni. Iodine And Zinc Balanced Equation.
From www.toppr.com
Balanced equation for the reaction between zinc and dilute sulphuric Iodine And Zinc Balanced Equation Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. Web zinc iodide = zinc + iodine. Zn (s) + i 2 (s) → no reaction In this experiment, you will react roughly equivalent masses of zinc and iodine to yield a simple binary compound. Web zn + i = zni2 is. Iodine And Zinc Balanced Equation.
From ceaqrhjq.blob.core.windows.net
Zinc Iron Iii Chloride Balanced Equation at Carolyn Smith blog Iodine And Zinc Balanced Equation Zinc powder is added to a solution of iodine in ethanol. Web zn + i = zni2 is a synthesis reaction where one mole of zinc [zn] and two moles of iodine [i] combine to form one mole of zinc iodide [zni. Zn (s) + i 2 (s) → no reaction This can be reversed using electrolysis to decompose the. Iodine And Zinc Balanced Equation.
From www.bartleby.com
Answered n. Write a balanced chemical reaction… bartleby Iodine And Zinc Balanced Equation Zni2 = zn + i is a decomposition reaction where one mole of aqueous zinc iodide [zni 2] decomposes into. Web zinc iodide = zinc + iodine. This can be reversed using electrolysis to decompose the compound. Web using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. In this experiment, you will react roughly equivalent. Iodine And Zinc Balanced Equation.
From www.toppr.com
In the reaction between zinc and iodine, zinc iodide is formed. Which Iodine And Zinc Balanced Equation Web the chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the. Zn (s) + i 2 (s) → no reaction Web the reaction of zinc metal with iodine shows direct combination, decomposition, recrystallization of sublimed i2, and electrolysis. Web zn + i = zni2 is a synthesis reaction where. Iodine And Zinc Balanced Equation.